Abstract
Introduction. Oligomonocytic chronic myelomonocytic leukemia (OM-CMML) is defined as those myelodysplastic syndromes (MDS) or MDS/MPN unclassifiable cases with relative monocytosis (≥ 10% monocytes) and a total monocyte count of 0.5-<1x10 9/L. These patients show similar clinical, genomic and immunophenotypic features than overt CMML, although the majority of them are currently classified as MDS following WHO 2017 classification. TET2 is the most prevalent mutated gene in CMML and it is frequent to find multiple TET2 mutations in the same patient.
Objective. We investigated TET2 mutations in 42 OM-CMML, their diagnostic value and their correlation with immunophenotypic pattern, and compared them with that observed in 54 CMML and 86 MDS.
Patients and methods. Samples were collected from 182 patients: 42 OM-CMML, 54 overt CMML, and 86 MDS. Molecular characterization was performed by next-generation sequencing (NGS) using a custom panel (QIAseq Custom DNA Panels, Qiagen) including the whole codifying region of 25 myeloid-associated genes and sequenced using Illumina technology. Multiparametric flow cytometry (FC) analysis of monocyte subsets was performed on whole PB collected on EDTA. Cell surface staining of 2 × 10 6 cells was performed and at least 500 000 total events were acquired per tube (FACS Canto II; BD Biosciences). The FC strategy analysis, the 5-tube experimental panel, and the NGS custom panel are described in Calvo & Garcia-Gisbert, Blood Adv 2020.
Results.
Patients with multiple TET2 (multi-TET2) mutations (2 or more) were identified with similar percentages in OM-CMML (17/42, 40.5%) and CMML (29/54, 53.7%) while they were infrequent in MDS (5/86, 5.8%) (P<0.001). In MDS patients, 4/5 with multi-TET2 showed a percentage of monocytes >10% though they were not considered OM-CMML since the monocyte count was below 500/microL. CMML and OM-CMML patients with multi-TET2 showed predominantly 2 TET2 mutations (CMML: 20/29, 69%; OM-CMML: 14/17, 82%;). The distribution of patients with >2 TET2 mutations was similar in CMML and OM-CMML. We did not observe differences among the frequency of nonsense, frameshift or missense TET2 mutations, nor between early or late-truncating (early: aa 1-1128, late: 1128-1936) TET2 mutations between OM-CMML and CMML.
TET2 variant allele frequency (VAF) was significantly lower in MDS (median 11.83%) than in OM-CMML and CMML (P<0.001 in both comparisons). OM-CMML and CMML showed similar TET2 VAF (medians 41.4% and 40.3%). In those patients with TET2 VAF >55% or a sum of muti-TET2 mutations VAF above 55% (17 OM, 19 CMML, 2 MDS), we could infer a biallelic alteration in the same clone (Coltro, Leukemia 2020). We did not observe differences in the frequency of OM-CMML showing biallelic TET2 mutations when compared to CMML.
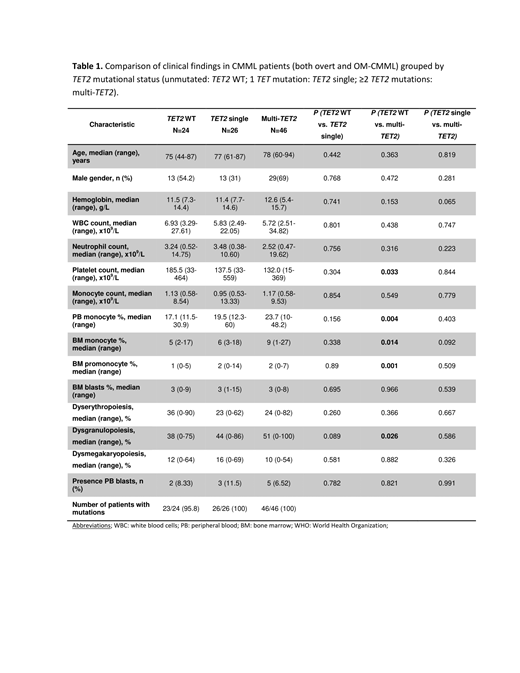
The main clinical characteristics of CMML patients (overt and OM-CMML), grouped by TET2 mutational status (unmutated: TET2 WT; 1 TET mutation: TET2 single; ≥2 mutations: multi-TET2) are depicted in Table 1. As shown, we did not observed significant differences when comparing TET2 single to TET2 WT or multi-TET2 Of note, multi-TET2 patients showed a higher percentage of PB and BM monocytes, BM promonocytes and more dysgranulopoiesis than TET2 WT patients.
Patients with TET2 WT presented a lower number of mutated genes than TET single and multi-TET2 patients (P=0.019 and P=0.029). In line with previous studies, IDH mutations and TET2 mutations where mutually exclusive (P=0.001). Co-mutation of SRSF2 and TET2, the well-accepted gene signature of CMML, was found in 25/96 patients (26.0%, 13 OM-CMML, 12 CMML) in 10 cases co-mutated with TET2 single and in 15 cases with multi-TET2.
We assessed the proportion of OM-CMML patients with MO1>94% since this has been shown as very sensitive and specific feature of CMML. In TET2 mutated OM-CMML, we observed a higher proportion of patients with MO1>94% (90% mut. vs 40% WT; P=0.001) and CD56 expression on monocytes (70% mut. vs 30% WT; P=0.025). We observed a trend (non-significant) difference in the proportion of patients showing these features when comparing TET2 single vs multi-TET2 OM-CMML (single, CD56: 57.1%, MO1>94%: 85.7%; multi-TET2, CD56: 81.3%, MO1>94%: 93.8%).
Conclusion. The high occurrence of multiple TET2 mutations in overt and OM-CMML and its infrequency in MDS is a new biological clue for supporting the consideration of OM-CMML in the continuum of CMML.
Salar: Celgene: Consultancy, Speakers Bureau; Gilead: Research Funding; Roche: Consultancy, Speakers Bureau; Janssen: Consultancy, Speakers Bureau. Bellosillo: Roche: Research Funding, Speakers Bureau; Qiagen: Consultancy, Speakers Bureau; Thermofisher Scientific: Consultancy, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal